Unit 1: Care Management Overview
5.1 Introduction to Care Management
5.1 Components of the Care Management Program
5.1 Access to Medical Management Services
5.1 Criteria for Medical Management Decisions
5.1 Criteria for Behavioral Health Services
5.1 Member Consent for Release of Medical Records
5.1 Practice Guidelines and Standards of Care for HIV (NY Only)
Care management incorporates a comprehensive integrated solution that encompasses all aspects of engagement and self-management by providing information, support, and interventions across the continuum of care.
The Highmark Care Management Program focuses on the integration of the delivery of health care services with our members, their employers or groups, and our network providers. It is designed to comply with all federal, state, and external review body regulations and standards.
Employer groups and individuals receive a core set of services including:
- Utilization Management (medical, behavioral health, and pharmacy)
- Wellness
- Condition Management
- Case Management/Care Transitions
- Care Coordination
The activities and functions are used to optimize appropriate utilization of health care resources within the appropriate settings, including acute inpatient, outpatient, outpatient imaging, home health care, skilled nursing, and rehabilitation.
Various Departments Involved in Coordination of Services
Highmark’s Clinical Services is directly responsible for implementation of the Care Management Program through its Utilization Management (UM) and Medical Management and Quality (MM&Q) departments. The staff consists of clinical, non-clinical, and administrative personnel who support the coordination and seamlessness of the services provided to the member. Highmark Behavioral Health Units are included within the scope of Clinical Services.
Within Clinical Services, the physician reviewers provide direction and oversight to the overall care planning process. They support the functions of the physician staff as well as the clinical staff.
Clinical Services’ goal is to deliver a comprehensive and integrated care management program that positively impacts both members’ health and medical benefit costs. Care managers may also manually refer members to case and condition management based on individual member needs.
Behavioral Health
Additional information on the utilization processes and procedures specific to behavioral health can be located in the Highmark Provider Manual Chapter 5 Unit 4: Behavioral Health. For requirements and guidelines for behavioral health providers, please see Chapter 4 Unit 2: Behavioral Health Providers.
Scope of Services
There are numerous components of the Care Management Program. These components are inclusive of both medical and behavioral health services.
The services listed below are integrated into Highmark's total Care Management Program. They include, but are not limited to:
- Health Information and Support
- Utilization Management
- Significant Medical Decision and Treatment Support
- Condition Management
- Maternity Education and Support
- Case Management
- Behavioral Health Case Management
- Coordination between Medical and Behavioral Health Management
- Prevention and Wellness
- Radiology Management
- External Review Services
- Medical Technology Assessment Reviews
Customized Care Management Programs
Employer groups may select from a set of core services or increase their depth of services by adding programs such as wellness coaching or by intensifying their condition/disease management program.
This allows employers to address their specific population, whether they have employees who will benefit from chronic illness intervention and education or employees who are interested in participating in wellness programs beyond what may be provided in a traditional worksite wellness program.
Partnerships Enhance Services
By partnering with vendors who provide expertise in specific care management services, Highmark enhances the services provided to members. These vendors work in coordination with Highmark to provide a seamless, integrated program for Highmark members.
eviCore Healthcare
eviCore healthcare (eviCore) is an independent specialty medical benefits management company that provides utilization management services for several of Highmark’s care management programs. These include:
- Radiation Therapy Authorization Program (DE, PA, and WV Only)
- Laboratory Management Program (DE, PA, and WV Only)
- Musculoskeletal Surgery and Interventional Pain Management Services Prior Authorization Program (DE, PA, and WV Only)
Additional information on all programs managed by eviCore can be found on the Provider Resource Center by selecting Policies and Programs then Care Management from the main menu.
Davis Vision
Davis Vision is our partner for routine vision exams, glasses, and contacts for Highmark members in New York. Routine vision benefits may vary by plan.
Coverage for problem eye services is considered a medical benefit and will not be managed by Davis Vision. Medical claims (for care including but not limited to infection, macular degeneration, glaucoma, detached retina) will continue to be submitted to Highmark electronically through Administrative Services of Kansas, Inc. (ASK) for all members.
Davis Vision Pediatric Exchange Vision Coverage
Pediatric (up to age 19) Affordable Care Act (ACA) members who are covered by our Individual and Small Group plans must see Davis Vision providers for routine eye exams, and eye care accessories (frames, lenses, contact lenses) as these benefits are embedded in the medical benefit.
If you care for pediatric ACA members, you will need to be in the Davis Vision network. Providers already contracted with Davis Vision can send claims to Davis Vision directly for routine eye exams and eye care accessories for these Highmark pediatric members.
Davis Vision Medicare Advantage Vision Coverage
All covered Medicare Advantage vision services and products claims must be processed through Davis Vision. This includes routine vision exams, glasses, contacts, and post-cataract benefits for glasses or contacts.
Members with Medicare Advantage HMO plans must use a Davis Vision participating provider to receive benefits for vision services. Members with Medicare Advantage PPO plans can see a non-participating Davis Vision provider; however, the claims must be submitted to Davis Vision using the Davis Vision’s Out of Network Claim form.
Filing Vision Claims with Davis Vision
If you are contracted with Davis Vision, you will be able to provide and bill Davis Vision directly for covered routine vision services to Highmark Commercial and Medicare Advantage members.
Non-Participating Davis Vision providers should not submit claims directly to Highmark using ASK for routine vision services including routine exam, glasses, contacts, or Medicare Advantage required post-cataract benefits.
Any problem or diagnosis-focused eye services that are considered medical claims (including but not limited to infection, macular degeneration, glaucoma, detached retina) should be submitted and billed directly to Highmark electronically through ASK.
Contacting Davis Vision
If you are already a part of the Davis Vision network and have a general inquiry, you can reach them at 800-773-2847. Call center hours of operation are Monday – Friday: 8 a.m. to 8 p.m. (Eastern time), and Saturday: 9 a.m. to 4 p.m.
Providers who are not currently part of the Davis Vision network and would like to join, can contact Davis Vision HERE.
Utilization management activities focus on opportunities to reduce clinically unnecessary variation in the delivery of services, to utilize clinically appropriate alternative levels of care, to assist with timely and effective discharge planning, to facilitate the appropriate use of benefits, and to proactively identify members who may benefit from other services such as health promotion and disease prevention programs, treatment decision support, chronic condition support, depression management services, and/or case management services.
The utilization management process incorporates a rules engine that automatically triggers referrals to case management and condition management based on a select group of diagnoses and procedures that are entered. Care managers may also manually refer members to case and condition management based on individual member needs. Components of the utilization management process are described below.
Authorization
An authorization is a determination by Highmark that a health care service proposed for or provided to a member is “medically necessary” as that term is defined by the member’s contract.
Prior Authorization
Prior authorization (also known as preservice review or precertification review) is the process by which services requiring authorization are evaluated against criteria for medical necessity and appropriateness prior to the receipt of services.
Inpatient Admissions
Requests for inpatient services are evaluated according to criteria for medical necessity, appropriateness, the most appropriate setting, and benefit availability. Authorization is required for all in-network and out-of-network inpatient services and required under all Highmark products whenever a member is admitted as an inpatient to any of the following facilities:
- Acute Care
- Long-Term Acute Care (LTAC)
- Inpatient Rehabilitation Facility
- Mental Health or Substance Abuse Treatment Facility
- Skilled Nursing Facility (SNF)
Concurrent Review
Concurrent review, also known as continued stay review, is the process for assessing and determining the ongoing medical necessity and appropriateness for an extension of services that have been previously authorized. Outpatient requests should be made at least 24 hours prior to the expiration of the original authorization period (last day of treatment).
Concurrent review is also conducted for all inpatient settings after the initial authorization has been obtained, including acute inpatient, LTAC, SNF, and inpatient rehabilitation. Requests for continued stay should be made no later than the last covered day.
Concurrent review is conducted for all behavioral health inpatient services, for medical care for select facilities based on the reimbursement structure, for medical services reimbursed by the visit, or for accounts with specific contract provisions.
Retrospective Review
Retrospective review (also known as post-service review) is the process of assessing the appropriateness of medical services rendered to a member after the service has been provided.
Network providers have an obligation to cooperate with preservice authorization review procedures. If the provider fails to comply, Highmark has the right to review the service retrospectively. If the service is deemed not medically necessary, then payment may be denied or recovered from the provider. Providers who consistently fail to request authorizations on a preservice basis may be subject to corrective action by the Credentials Committee.
Clinical Review Process
Initial reviews of authorization requests are performed by registered nurse reviewers with clinical experience. They utilize MCG Care Guidelines, Highmark or Medicare Advantage medical policies, and other clinical criteria to review the medical necessity of the requested services.
The nurse reviewer may authorize a service that meets criteria. Reviewers have access to consult with a medical director. If an initial reviewer is unable to approve a service, the case is referred to a physician medical director or other physician reviewer. The physician will evaluate the request using Highmark’s criteria and considering the specific clinical aspects of the individual case. Only a physician may determine that a service is not medically necessary.
Medical necessity reviews by Highmark medical directors and other clinical staff do not constitute medical advice or treatment, nor do they create any provider-patient relationship. Such reviews are solely for the purpose of determining whether services meet Highmark criteria for medical necessity, which is a condition for services to be covered and reimbursable.
Peer-to-Peer Conversation
Highmark provides the opportunity for a treating physician to discuss the denial of an authorization with the medical director or other physician reviewer who made the determination. The purpose of the peer-to-peer conversation is to allow the ordering or treating provider an opportunity to discuss the case directly with the reviewer and to provide any additional information or perspective that may be helpful, prior to initiating a formal appeal.
This discussion may help resolve the issue and spare the time and expense of an appeal. Highmark will advise the treating provider of the availability of this process when verbally notifying the provider of an authorization denial (if a peer-to-peer conversation has not already occurred).
The provider may initiate the peer-to-peer discussion by calling Clinical Services.
For More Information
For additional information on initiating a peer-to-peer conversation, please see the Highmark Provider Manual Chapter 5 Unit 5: Denials, Grievances, and Appeals.
Discharge Planning
Discharge planning is a proactive and collaborative process between the provider and the Care Manager or Health Coach and is an integral part of the inpatient review process, often beginning prior to a scheduled admission and continuing throughout the course of treatment. Members receiving inpatient acute, rehabilitation, and skilled nursing services are followed at specific intervals throughout the admission to anticipate and identify needs, quality of care concerns, gaps in care, and/or barriers to care.
Behavioral Health
Highmark has a dedicated behavioral health unit staffed by behavioral health professionals and registered nurses with significant clinical behavioral health experience. The behavioral health case managers review authorization requests and referrals for behavioral health services. Case managers have access to Highmark medical directors and consulting psychiatrists for consultation on individual cases.
For more information on behavioral health authorizations, see the Highmark Provider Manual Chapter 5 Unit 4: Behavioral Health.
For providers in all regions, Availity Essentials is key to Highmark’s utilization management services. It is provided cost-free to Highmark network participating providers and can be used for submitting most authorization requests. Other functionality available on the platform related to utilization management includes discharge planning, referrals to Case Management, and inquiry functions to confirm status of your authorization requests.
When Availity is not available or for non-routine inquiries that cannot be handled through Availity, Clinical Services may be contacted.
When rendering a medical necessity determination, Clinical Services uses medical necessity criteria that are based on sound medical and clinical evidence. The criteria used are formally reviewed annually and revised as necessary
In addition to nationally recognized evidence-based criteria, Highmark medical policies are used that consider regional and local variations in medical practice. Procedures are also in place for applying criteria based on individual needs.
Definition: Medically Necessary
Except where any applicable law, regulation, or government body requires a different definition (i.e., the Federal Employees Health Benefits Program, Highmark’s Healthy Kids [CHIP], Centers for Medicare & Medicaid Services [CMS] as to the Medicare Advantage program, etc.), Highmark has adopted a universal definition of medical necessity.
The term medically necessary, medical necessity, or such other comparable term shall mean health care services or supplies that a provider, exercising prudent clinical judgment, would provide to a patient for the purpose of preventing, evaluating, diagnosing, or treating an illness, injury, disease, or its symptoms, and that are:
- In accordance with generally accepted standards of medical practice; and
- Clinically appropriate, in terms of type, frequency, extent, site, and duration, and considered effective for the patient’s illness, injury, or disease; and
- Not primarily for the convenience of the patient, physician, or other health care provider, and not more costly than an alternative service, sequence of services, or site of service at least as likely to produce equivalent therapeutic or diagnostic results given the nature of the patient’s diagnosis, treatment, illness, injury, or disease, the severity of the patient’s symptoms, or other clinical criteria.
Generally Accepted Standards of Medical Practice
For these purposes, generally accepted standards of medical practice means standards that are based on credible scientific evidence published in peer-reviewed medical literature generally recognized by the relevant medical community, Specialty Society recommendations, and the views of providers practicing in relevant clinical areas and any other relevant factors.
Request for Criteria
Highmark uses resources such as nationally recognized clinical review criteria, medical policy, and Medicare guidelines in determining whether a requested procedure, therapy, medication, or piece of equipment meets the requirements of medical necessity and appropriateness. This is done to ensure the delivery of consistent and medically appropriate health care for our members.
To assess a request for medical necessity and appropriateness, relevant clinical information is reviewed. When the clinical information is incomplete, the Utilization Management department attempts to obtain the relevant clinical information from the provider and/or facility or by obtaining the member’s medical record. A medical necessity determination is made by either a Utilization Management Clinician, Pharmacist, or Medical Director after the available clinical information has been reviewed.
At any time, the PCP or specialist may request a copy of the criteria/guidelines used in making medical necessity determinations by calling Highmark at:
- 800-421-4744 for Medical/Surgical related criteria/guidelines
- 800-258-9808 for Behavioral Health related criteria/guidelines
- Pharmacy-related criteria/guidelines
- Delaware, Pennsylvania, and West Virginia: 800-600-2227
- New York: 877-698-0793
Criteria Used
The Medical Utilization Management staff uses the following criteria, guidelines, and policies:
- MCG Care Guidelines
- Highmark Medical Policy
- Medicare Advantage Medical Policy
- American Society of Addiction Medicine (ASAM) criteria
- New York Only: Level of Care for Alcohol and Drug Treatment Referral (LOCADTR) criteria
MCG Care Guidelines
The MCG Care Guidelines provides criteria for settings ranging from acute through outpatient. Care managers base medical necessity decisions for adult and pediatric acute, long-term acute, sub-acute and skilled nursing facility (SNF), rehabilitation, and home care services on the MCG Care Guidelines. The MCG Care Guidelines are embedded in Predictal, accessible via Availity.
Utilization Decision Making
Highmark makes utilization review decisions based only on appropriateness of care and service and the existence of coverage. Such reviews are solely for the purpose of determining whether services meet Highmark criteria for medical necessity and are being delivered in the most appropriate setting, which are conditions for services to be covered and reimbursable.
They do not reward practitioners, providers, Highmark employees, or other individuals conducting utilization review for issuing denials of coverage or service, nor do they provide any financial incentives to utilization management decision makers to encourage denials of coverage.
Highmark Medical Policy
Highmark's Medical Policy guidelines address both clinical and claim payment reimbursement issues. These policies are developed and maintained in accordance with national standards such as those set by the National Committee for Quality Assurance (NCQA).
The Medicare Advantage Medical Policy guidelines are based on national coverage determinations issued by the Centers for Medicare & Medicaid Services (CMS) and local coverage determinations established by Novitas Solutions, Inc. in Pennsylvania and Palmetto GBA in West Virginia.
Highmark Medical Policies are available on the Provider Resource Center under CLAIMS, PAYMENT & REIMBURSEMENT. Medicare Advantage Medical Policies are available on CMS’ Medicare Coverage Database website.
Additional information regarding Medical Policy may also be found in the Highmark Medical Policy section of this unit.
Clinical Judgement
Please note that the use of these and other guidelines requires, and never replaces, clinical judgment.
Criteria Review
All criteria are reviewed, approved, and/or revised at least once annually by the Care Management & Quality Committee (CMQC). The CMQC is comprised of practicing physicians in the community and physicians in hospital administrative positions who are involved in care management functions.
Important: FEP Medical Policies
Federal Employee Program (FEP) medical policies are specific to FEP benefits and may differ from Highmark’s medical policies; however, in the absence of FEP medical policy, consult Highmark medical policy for guidance.
To view FEP medical policies in their entirety, please refer to the Federal Employee Program’s website. From the homepage, scroll down to the footer and select Policies & Guidelines.
Policies are not intended to be prescriptive; thus, medical policy is not an authorization, certification, explanation of benefits, or a contract. Benefit eligibility and application are determined by the Federal Employee Program.
Basis of Authorization Decisions
Highmark's Behavioral Health Unit bases its decisions to authorize care on available clinical information, availability and appropriateness of less restrictive treatment settings, appropriate medical necessity criteria, the member’s benefits, and the safety of the patient and others.
Criteria
With the exception of substance abuse treatment, Highmark's Behavioral Health Unit applies MCG Care Guidelines when reviewing the medical necessity and appropriateness of behavioral health services.
Highmark's Behavioral Health Unit uses the current version of the American Society of Addiction Medicine (ASAM) Criteria when reviewing the medical necessity of substance abuse treatment.
Level of Care for Alcohol and Drug Treatment Referral (LOCADTR) Criteria
New York State Office of Addiction Services and Supports (OASAS), in partnership with National Center on Addiction and Substance Abuse at Columbia University (CASA Columbia), developed the Level of Care for Alcohol and Drug Treatment Referral (LOCADTR) 3.0, a web-based tool to assist substance abuse treatment providers, Medicaid Managed Care plans, and other referral sources in determining the most appropriate level of care (LOC) for a client with a substance use disorder and/or problem gambling disorder. This tool enables the referral source to identify the most appropriate treatment setting closest to the client's community.
For More Information
Please see the Highmark Provider Manual Chapter 5 Unit 4: Behavioral Health for additional information on medical necessity criteria for behavioral health services.
Medical policies are documents that provide medical necessity and coverage guidelines for all our medical-surgical products, including managed care. These guidelines address hundreds of medical issues including diagnostic and therapeutic procedures, injectable drugs, and durable medical equipment. Highmark’s Medical Policy guidelines have been integrated into the claims processing system, which allows for cost-effective claims processing and ensures accurate administration of our members’ health care benefits.
In addition to medical policies for our commercial products, Highmark also maintains medical policy guidelines for our Medicare Advantage products. See the Highmark Provider Manual Chapter 5 Unit 3: Medicare Advantage Procedures for additional information.
Policy
Medical policies do not constitute medical advice, nor are they intended to govern the practice of medicine. They are merely intended to reflect Highmark’s coverage guidelines. Coverage for services may vary for individual members based on the terms of the benefit contract.
Highmark retains the right to review and update the medical policy guidelines in its sole discretion. These guidelines are the proprietary information of Highmark. Any sale, copying, or dissemination of the medical policies is prohibited; however, limited copying of medical policies is permitted for individual use.
Medical Policy Development
Highmark continually reviews its existing medical policies to ensure that they reflect evidence-based medicine, the current standard of care, and the appropriate place of service. Highmark’s Medical Policy Department ensures that medical policies are developed and maintained in accordance with national standards such as NCQA and the Blue Cross and Blue Shield Association. For Highmark Medicare Advantage products, the Centers for Medicare & Medicaid Services (CMS) requires that Medicare Advantage insurers use CMS national policy and the regional Medicare B Carrier’s local policy.
To begin the process of adding or revising its policy guidelines, Highmark’s Medical Policy department reviews published, peer-reviewed medical literature along with information and determinations from multiple sources — including the Food & Drug Administration (FDA) and professional medical societies.
After the Medical Policy department has performed its initial research, it may solicit opinions from appropriate health care professionals. If the procedure in question is performed by a particular specialty, consultants within that specialty may be contacted, or the issue may be referred to one of specialty subcommittees for review and recommendation regarding a medical policy coverage position. The subcommittees include but may not be limited to Cardiology, Gene and Cell Therapies, Musculoskeletal, Neurosciences, Pediatrics, and Radiation Oncology. To develop a draft policy, the Medical Policy department utilizes input from all these sources.
Once the policy is drafted, the Medical Policy department then collaborates with the Clinical Policy Management Committee (CPMC) in making a final determination on the policy prior to publication. The CPMC consists of staff Medical Directors working under the direction of Highmark’s Chief Medical Officer.
Once the policy is drafted, the Medical Policy department then collaborates the New York Medical Management Clinical Committee (MMCC) for review and voting and then to the QMC and Board of Directors for approval.
Provider Involvement
Health care professionals play an important role in Highmark’s Medical Policy development. They provide medical expertise that helps in the development of coverage guidelines.
In addition, independent health care professionals are active in a variety of positions that influence the core of Highmark’s operations. They make up the majority of committees that help to define medical policy, resolve claims disputes, and promote the delivery of quality medical care to Highmark members.
Place of Service Requirements
Highmark develops medical policy as the foundation for determining coverage eligibility for certain health care services rendered to its members. Highmark continually reviews its existing medical policies to ensure that they reflect evidence-based medicine, the current standard of care, and the appropriate place of service. Place of service requirements are indicated on select commercial medical policies to clearly define the most appropriate setting for specific services.
If place of service requirements apply, the medical policy will include a Place of Service section. Additional policy guidelines are also listed under this heading, if applicable.
Note: Place of service requirements do not apply to Highmark's Medicare Advantage business, which is governed by regulations and policies developed and promulgated by CMS.
Determining Medical Policy Criteria
Facilities must coordinate with the ordering and/or performing provider before the date of service. The facility should work with the ordering and/or performing provider, as necessary, to ensure medical policy criteria are met. Alternatively, the facility can initiate an inquiry through the Provider Service Center if there are concerns about whether the facility services to be performed meet applicable Highmark medical policy criteria.
Accessing Highmark’s Medical Policies
Highmark’s commercial medical policies are accessible on the Provider Resource Center under Policies and Programs and then Medical Policies.
Medical policy may differ in service areas based on state regulatory requirements. Please be sure to access the appropriate medical policies from the Provider Resource Center for your service area and/or based on the member’s coverage.
Note: Highmark medical policies online are considered to be current; however, users can access and review terms of previous versions of a policy prior to the effective date of the current version.
Notification of New Policies and Updates to Existing Policies
Our monthly Medical Policy Update newsletter provides advance notification of new policies and upcoming changes to existing medical policies. You can find current and past issues of Medical Policy Update on the Provider Resource Center. In addition, you can sign up for our mailing list and receive a monthly email notification when the latest issue of Medical Policy Update is published. To subscribe, select Join Our Mailing List from the top right corner of the PRC and complete the form.
Important
New medical policies and updated versions of existing medical policies are not available for viewing until the effective date of the policy, or the following Monday if that date falls on a weekend or holiday.
Claim Impacts Based on Application of Medical Policy
Although claims for services impacted by Highmark medical policy may be paid when submitted, Highmark reserves the right to review such cases retrospectively to ensure that payments made were appropriate based upon the applicable medical policy requirements. Complete and careful documentation must be maintained in the member's medical record in case of any such post-payment review.
If it is determined that Highmark medical policy requirements were not met in a particular case and, therefore, a service is not eligible for coverage, the payment Highmark has made for the services will be retracted. As always, if the facility disagrees with the result of such a review, it can appeal the decision. In the event of a conflict between the requirements of the Highmark Provider Manual and Medical Policy, the following order of control should apply: a) First, Medical Policy; (b) Second, the Highmark Provider Manual.
Important: FEP Medical Policies
Federal Employee Program (FEP) medical policies are specific to FEP benefits and may differ from Highmark’s medical policies; however, in the absence of FEP medical policy, consult Highmark medical policy for guidance.
To view FEP medical policies in their entirety, please refer to the Federal Employee Program’s website. From the homepage, scroll down to the footer and select Policies and Guidelines.
Policies are not intended to be prescriptive; thus, medical policy is not an authorization, certification, explanation of benefits, or a contract. Benefit eligibility and application are determined by the Federal Employee Program
Non-covered services include services ineligible under the member’s plan documents, deemed experimental or investigational, or deemed not medically necessary by Highmark.
Except otherwise stated herein, a provider may always bill a commercial member for non-covered services if the provider has given the member advance written notice that the service(s) may not be eligible for coverage and an estimate of the cost thereof. Thereafter, the member must agree in writing to assume financial responsibility for the service(s) in advance of receiving such service(s). The signed agreement shall be kept in the provider’s records.
Member Desire to Obtain Medically Unnecessary Services
On occasion, situations may arise where Highmark determines, in advance of a service being provided, that the service is not medically necessary, yet the member still desires to obtain the service and is willing to bear the cost. The provider may bill the member for such services only if:
- The provider has requested a determination of medical necessity from Highmark in advance of providing the service and Highmark determines in writing that the proposed service is not medically necessary;
- The provider informs the member of Highmark’s determination in writing and in advance of providing the service; and
- The member indicates in writing that he or she understands and agrees that he/she will be totally responsible for paying for the service and is waiving all rights to submit a claim to Highmark.
The documentation requirements for appropriate billing for non-covered services and non-medically necessary services detailed in this section cannot be met with a blanket financial responsibility form in which the patient agrees to be financially responsible for any charges not paid by insurance. The documentation must: (i) describe the specific service in question; (ii) state clearly that Highmark has determined that the service is not medically necessary; and (iii) clearly document the patient’s agreement to be personally responsible for payment and not to submit a claim to Highmark.
By this process, Highmark acknowledges that, in limited circumstances, a member may want to enter a private arrangement with a network provider to obtain and pay for a service, knowing that the service is not reimbursable under the member’s coverage with Highmark. Highmark will not preclude the provider from billing the member in these special circumstances as long as the written documentation is prepared in advance of the service to demonstrate that the member entered into the arrangement knowingly and with full knowledge of the financial consequences.
Medical records are requested by Highmark when it does not have the information needed to determine the medical necessity and appropriateness of the services being provided.
When Medical Records Are Requested
If Highmark does not have sufficient information to determine whether services are medically necessary and appropriate, medical records will be requested. Medical records can be requested for either medical or behavioral health services, and for either inpatient or outpatient services.
The medical record requests are made in writing. The request is addressed to the attention of the hospital’s Medical Records Department.
Minimum Necessary Standards
Medical record requests will be limited to only the minimum necessary amounts of personal health information (PHI) needed to accomplish the intended purpose for which the PHI is being requested, used, or disclosed.
Confidentiality
In accordance with applicable regulatory and accrediting body requirements, as well as Highmark corporate policy, all personally identifiable confidential information obtained to manage a member’s care is maintained in such a manner as to protect the privacy of all individuals.
Provider Responsibility for Timeliness
Regulatory standards require health plans to make medical necessity decisions within strict time frames. In some cases, the regulatory standard does not provide additional time for obtaining medical records.
For this reason, it is important for providers to provide all relevant medical records within the time frame stipulated in the written request. Lack of response or a late response to the request for medical records may result in a denial of payment.
Non-Reimbursement Policy
According to Highmark policy, Highmark does not reimburse participating network providers for supplying medical records to Highmark.
BlueCard® Requests
On occasion, Highmark may request medical records for an out-of-area member in the BlueCard Program who has received services from you. The request is made in writing via a standard Medical Records Request Form.
Please respond to these requests as quickly as possible. The Blue Cross Blue Shield Association (which sponsors the BlueCard Program) encourages a response time frame of ten days or less. Your prompt return of medical records helps to expedite the review process and avoid unnecessary claim denials.
When mailing medical records, please attach/enclose the original Medical Record Request Form. This helps to ensure that the records reach the individual who requested them.
Note: For additional information for medical record requests for out-of-area BlueCard members, refer to the Highmark Provider Manual Chapter 2 Unit 6: The BlueCard Program.
Routine Situations
As a HIPAA-covered entity, Highmark has established the following policy regarding routine member consent:
Highmark's policy is that it will not request or obtain consent of its members in connection with the use or disclosure of protected health information (PHI) for treatment, payment, or health care operations. Under certain limited situations, Highmark may elect to obtain consent from a member.
Non-Routine Situations
For certain situations, a member may be asked to sign an authorization to use or disclose specific PHI. This includes information related to any of these topics:
- Psychotherapy notes
- Substance abuse
- Sensitive diagnoses such as HIV, STIs, or AIDS
When asked for medical records of this nature, the facility is responsible for obtaining the authorization from the member and submitting it to the Clinical Services department with the requested records.
Highmark’s Health Engagement Operations department is responsible for case management services, offered at no cost to Highmark members. Case management is a systematic, proactive, and collaborative approach to effective assessment, monitoring, and evaluation of options and services required to meet an individual member’s needs for health care services.
Case management is a collaborative process involving the physician, the patient and his or her support system, our Case Managers, and other health care service providers to encourage and assist patients to achieve their optimum level of health, self-management, and social and occupational functioning.
Purpose
Case management is offered to assist members who have complex or high-cost health care needs. Its purposes are:
- To help determine the health care needs of the patient; and
- To help plan for and coordinate the provision of needed services through communication, education, and the use of available resources, to achieve jointly established short and long-term goals.
Activities include assessment, planning, facilitation, advocacy, communication, and education to help the member meet his or her health care needs. Case managers can also help protect the welfare and safety of members through identifying and reporting risks of abuse, violence, and suicide. Case management can also assist members to understand their benefits and other consumer protections including medical directives and power of attorney.
Provider Referrals for Case Management
Highmark encourages providers to identify members who could benefit from coordinated case management services. Providers can initiate a referral for case management services by utilizing Availity.
Please consider the following conditions for case management referral:
- Patients with multiple medical or behavioral health concerns or services
- Patients who lack a consistent caregiver, have financial concerns, and require community resources
- Patients with a life-altering diagnosis or condition such as brain trauma, cancer, or debilitating neurological condition
- Patients with difficulty achieving self-management resulting in frequent emergency room visits or hospital admissions
Identifying Members for Case Management
Highmark’s Case Management staff uses clinical, utilization, and predictive modeling/Member Listening System (MLS) indicators to identify members who could benefit from case management. The indicators include, but are not limited to, the following:
- High-risk diagnoses
- Complex disease processes
- Catastrophic medical events
- High-cost cases
- Complications of care
- Situational and discharge planning needs
- Psycho-social issues
- Financial issues
- Complex care coordination needs
- Multiple admissions and readmissions
- Health risk assessment screening
Screening and Consent
Members identified as potential candidates for case management are further screened for case management eligibility and are then contacted to discuss enrollment in the voluntary program. If a member qualifies, a Highmark Case Manager is assigned and will contact the member, and their family, if necessary, to initiate case management. Members initially declined for case management may be reconsidered if their clinical condition changes.
Case Manager Responsibilities
The Case Manager is responsible for the following:
- Contacting the member and his/her providers when appropriate
- Conducting a comprehensive assessment of the member’s needs
- Identifying the issues and/or barriers affecting the member’s care
- Developing, implementing, coordinating, and evaluating a plan of care in collaboration with the member and his/her providers
Case Managers have access to Highmark physician medical directors and other consulting physicians who can assist in the review of and planning for individual cases, conditions, and services.
Case Management Services
Highmark Case Managers are licensed registered nurses (RNs) or licensed behavioral health professionals able to assist your patient by providing services including, but not limited to, the following:
- Assessment of knowledge deficits regarding their condition, treatment, or benefit issues
- Reinforcement of educational information as directed by the physician or service provider
- Evaluation and reinforcement of medication use and adherence which is particularly important in polypharmacy situations
- Evaluation and reinforcement of adherence with treatment regime, including development of short- and long-term goals
- Intervention to assist with obtaining medical supplies or equipment
- Coordination of services among providers
- Communication of adverse situations to the physician or service provider
- Evaluation and assistance with financial concerns
- Information related to available community resources such as self-help support groups and other similar services
5.1 Condition Management
Condition Management Overview
Condition management programs focus on creating better outcomes of members identified with chronic illnesses by improving their self-management skills and understanding of their illness and treatment options.
Once members choose to participate, they are enrolled in a program specific to their needs. Members may provide consent to allow the Case Manager to discuss their condition with their caregivers.
Available Programs
Members may be identified for several different condition or case management programs. See the Reference Guide of Highmark Member Programs for a complete listing of available programs.
Availability of Case Managers
Case Managers are available to receive inbound calls 24 hours a day, seven days a week.
Case Managers are available for outbound calls to members from 8:30 a.m. until 9:00 p.m. Monday through Friday, and on weekends when requested by the member.
Case Managers are available for outbound calls to members from 8:30 a.m. until 6:00 p.m. Monday through Friday, and on weekends when requested by the member.
Primary Goals of Condition Management
Highmark's condition management programs focus on the following goals:
- Improving the quality of care and outcomes for members with chronic illnesses by addressing and closing gaps in care and improving their self-management skills;
- Improving member decision-making skills, including understanding of their treatment options in the context of their personal values, preferences, and priorities;
- Promoting dialogue and communication between the provider and member;
- Reducing clinical progression of conditions by encouraging preventive screenings and immunizations; and
- Reducing potentially avoidable healthcare costs and enhancing the provider's ability to provide high-quality, evidence-based care.
Enhanced Community Care Management (ECCM) is a non-billable service currently offered for Highmark Medicare Advantage, Highmark Individual ACA, Highmark Small Group, and HealthWay (Highmark employees with Highmark insurance) members to support the most complex and vulnerable members. The goal of the program is to help our members live their best lives possible while maintaining their independence in the community.
What is the ECCM Program?
ECCM’s interdisciplinary care team, including physicians, advanced practice providers (Nurse Practitioners or Physician Assistants), nurses and social workers, are all trained in motivational interviewing, health literacy, and serious illness conversations. For members who have advanced illness and need Supportive and Palliative Care, the interdisciplinary team will:
- Create and document a whole-person-advance care plan (centered on the member and their family)
- Help with pain and symptom management through medical and non-medical interventions in coordination with the member’s Primary Care Physician (PCP)
- Provide more frequent check-ins
- Help with care plan support, including monitoring of conditions and when to take medications
- Provide the family caregiver with education, counseling, and/or respite
- Assist with decision making, clarifying care priorities, and helping to match treatment and services to the member’s goals
- Assist with social determinants of health
- Attend to palliative care needs and help to relieve the symptoms and stress of a serious illness. The goal is to improve quality of life for both the patient and their family.
ECCM is a free, flexible program that reduces disruption for the member, family, and caregiver by streamlining communication across health care settings to ensure the member’s needs are matched with the appropriate resources. The team also provides closer oversight of the member and their illness (through virtual and in-home care – including nursing facilities) while working with the member’s doctor and health care providers.
How Does ECCM Differ from Hospice?
Palliative care strives to alleviate discomfort and pain to improve the quality of life for members. Palliative care can be provided during any stage of an illness and most frequently to members with life-limiting illness. It can be provided at the same time as curative care.
The table below outlines the differences between the palliative care services provided by hospice and services provided through ECCM.
|
Hospice… |
ECCM… |
|---|---|
|
Focuses on controlling pain and symptoms for those who no longer seek curative treatment or for whom treatment to prevent the progression of illness is no longer appropriate. |
Are primarily consultative with focus on controlling pain and symptoms, providing emotional support, facilitating decision-making related to care, and coordinating services while the member may still be receiving curative treatment. |
|
Is available when life expectancy is six months or less. |
Is available whenever the member needs it. |
|
Is a Medicare benefit. Medicare-covered services related to the member’s terminal condition and also medical services unrelated to the terminal condition are paid under Medicare when the member is in an active hospice election period. While in an active hospice election period, the member is not eligible for ECCM. |
Services are covered under the member’s benefits. Members are not eligible for ECCM if they are in an active hospice election period. |
Note: While a member is in an active hospice election period covered under traditional Medicare, the member’s benefit will continue to cover supplemental or extra benefits, such as vision and dental, which are not covered by Medicare.
Hospice Services
All Medicare-certified hospice providers will be able to continue providing hospice care to Highmark members. The ECCM program does not provide hospice services; however, the program refers its members who elect hospice services to local hospice providers. Members and their caregivers will continue to have their choice of hospice providers when they elect hospice care.
Additional Information And Referrals
Additional information on the ECCM Program is available on the Provider Resource Center – select Care Management Programs from the main menu.
Providers can refer members to the ECCM Program services through one of the channels below:
- Epic: Ambulatory Referral to ECCM
- Phone: 844-438-3226 (844-GET-ECCM)
- Fax: 844-978-2756
- Email: eccmreferrals@highmark.com
Members may contact Member Services at the number on the back of their ID cards for information on ECCM participation.
Benefits for Physicians, Mothers, and Their Babies
The High-Risk Maternity Program provides comprehensive care, support, and education for pregnant women and their babies, from pregnancy confirmation through the postpartum period and beyond, as needed. This program helps ensure all eligible members receive needed services and benefits. Enrollment begins with a prenatal assessment; high-risk pregnancies receive targeted prenatal education and interventions.
The High-Risk Maternity clinical practice guidelines are included in the Prenatal/Perinatal Care Preventive Health Guidelines, which are available on the Preventive Health Guidelines page of the Provider Resource Center.
Newborn Education Component
New mothers may not recognize the basic signs of illnesses in their babies, simply because they lack the necessary education, experience, and materials. As a result, these moms frequently take their newborns to the emergency room when home care was all that was needed.
By providing newborn care information to new moms, we hope to:
- Teach moms how to recognize signs of illness in their babies.
- Help them to better communicate with their baby's pediatrician.
- Avoid unnecessary trips to the emergency room.
- Reinforce proper preventive care and immunization schedules with moms.
Fact Sheet
We offer two maternity programs to support expectant mothers: The High-Risk Maternity Program for pregnancies identified as high-risk, and Baby BluePrints for low-risk pregnancies. All pregnant members should register during their first trimester, enrolling in the appropriate program based on their risk assessment. Both programs provide educational support and materials. The High-Risk Maternity Program includes case management to reinforce physician recommendations and enhance patient education. Postpartum newborn care education is offered to all new mothers.
The Four Major Components of the High-Risk Maternity Program
The High-Risk Maternity Program's four major components are:
- Enrollment of pregnant patients in the program
- Prenatal education for all patients
- Interventions for high-risk patients (including care coordination)
- Health education for newborn care
Enrollment by Physician
Physicians can refer pregnant members to the High-Risk Maternity Program via Availity. Members with low-risk pregnancies can self-enroll in Baby BluePrints by phone, calling 866-918-5267.
Prenatal Education
The program emphasizes the importance of early and ongoing prenatal care. Members receive information about their insurance benefits to ensure they are utilizing available benefits during and after pregnancy. In addition, education regarding importance of postpartum care and follow-up are reviewed.
Interventions for High-Risk Patients
Patients who are identified as high-risk on the prenatal assessment form will be evaluated. The nurse will act as a liaison between you and your patient by reinforcing your care instructions and offering patient education.
HIV Services
HIV pretest counseling should be provided to all prenatal clients. If a woman is found to be HIV positive, the clinician ordering the HIV test is responsible for arranging for a follow-up appointment to an HIV specialist or designated Al DS Center.
Department of Health Memorandum DOHM (Al 99-01) is the standard of care for HIV services and will:
- Provide all pregnant women with HIV counseling and education;
- Offer the pregnant woman confidential HIV testing; and
- Provide the HIV positive woman and her newborn infant the following services or make the necessary referrals for these services:
- Management of the HIV disease.
- Case management to assist in coordination of necessary medical, social, and addictive services.
Universal Recommendation for Testing of Pregnant Women
New York's regulatory framework for preventing mother-to-child transmission (MTCT) of HIV has proven highly effective and remains unchanged, except for the 2017 update that removed the requirement to obtain written or oral consent for HIV testing.
All pregnant women must be offered HIV testing as a clinical recommendation as early as possible during pregnancy. Third trimester testing is recommended for all pregnant women in New York State (NYS) who tested negative for HIV earlier in their pregnancy.
When being offered HIV testing, the woman should be provided the key points of information and informed of her right to decline the test. Pregnant women who are diagnosed as living with HIV should be linked to treatment as soon as possible to protect their health and prevent transmission of HIV to the newborn.
Women who present to the labor/delivery setting with no history of HIV testing during their current pregnancy should be counseled with the recommendation for HIV testing. If the mother declines testing in labor/delivery, the mother should be informed that her newborn will be tested immediately at birth without her consent. All newborns, including those tested at birth, are routinely tested for HIV through the New York State Newborn Screening Program.
Documentation of the woman's prenatal HIV testing should be forwarded to the delivering hospital and a copy of the mother's HIV test history results should be placed in the newborn's medical record to ensure administration of medications during labor/delivery and initiation of medication to the infant for the first four-six weeks of life or until the infant is definitively excluded from HIV infection.
To access the latest regulations, visit:
Acute HIV Infection During Pregnancy
The acute HIV infection in pregnancy guidelines recommend the following:
- Confirmation of preliminary positive expedited HIV test results.
- Vigilance for acute HIV infection in pregnant women who present with a compatible clinical syndrome, even if a previous HIV antibody test during current pregnancy was negative.
- Evaluation for acute HIV infection in pregnant or breastfeeding women who present with a febrile "flu" or "mono" like illness, or rash that is not otherwise explained.
- Immediate screening for suspected acute HIV infection by obtaining an HIV serologic screening test in conjunction with a plasma HIV RNA assay (a fourth-generation HIV antigen/antibody combination test is the preferred serologic screening test, if available).
- Repeat HIV RNA testing from a new specimen to confirm the presence of HIV RNA if HIV RNA or antigen was detected in the absence of HIV antibody.
- Baseline genotypic testing and initiation of ART while waiting for the results of resistance testing.
For HIV-positive women, documentation should reflect receipt of appropriate care.
Labor and Delivery
- Testing should be offered during labor and delivery for those who do not have documented third trimester HIV test results.
- Expedited testing of pregnant women who present for delivery without documentation of a negative HIV test should be made available.
Partner Services and the Role of Partner Services Programs
Medical providers or their designee must explain to all newly diagnosed patients the importance of notifying any sexual or needle-sharing partners that they may have been exposed to HIV. Partner services is a cornerstone of HIV prevention efforts that provides an opportunity for sexual or needle-sharing contacts of a person living with HIV to be offered testing in a timely manner, and if diagnosed with HIV infection, be linked into care. Every physician or other person authorized to order diagnostic testing is required to report HIV and AIDS diagnoses to the health department. This report must include identifying information about any contacts known to the clinical provider or provided to the clinical provider by the patient.
The HIV/AIDS Provider Portal may be used to report cases (including partners) and to request assistance from the health department with partner notification. As part of post-test counseling, the following must be provided to the patient:
- An explanation of the importance of notifying sexual or needle-sharing partners to prevent further transmission, and to promote early access of exposed persons to HIV testing, health care, and prevention services;
- A description of notification options and assistance available to the protected individual;
- A discussion about the risk of domestic violence and screening for domestic violence prior to partner notification in accordance with New York State Department of Health (NYSDOH) domestic violence screening protocol;
- The fact that known contacts, including a known spouse, will be reported to the health department. That protected persons will also be requested to cooperate in contact notification efforts of known contacts and that protected persons may name additional contacts they wish to have notified with the assistance of the provider or authorized public health officials; and
- An explanation that the name and other information about the person living with HIV will be protected during the contact notification process.
The NYSDOH Partner Services Program and the New York City (NYC) Health Department Contact Notification Assistance Program (C-NAP) provide a wide range of services including performing notifications; assisting patients with decision-making; and consulting with health care providers.
In some situations, Partner Services Specialists can meet with the patient at the same time that the laboratory results are given to assist with post-test counseling and development of a partner notification plan. Additional NYSDOH/NYC Department of Health and Mental Hygiene (NYCHMH) services may be available such as assistance in locating persons who test positive but who do not return for their results.
For more information about partner services and how to contact partner services programs throughout NYS, click HERE.
Important
In recognition of the need for ongoing partner services beyond the time of initial diagnosis of HIV, the 2016 updates to the NYSDOH Regulations formally prioritized partner services for people previously diagnosed with HIV who are at elevated risk of transmitting the virus to others. Several factors are considered as evidence of elevated risk of transmitting the virus to others. Those factors include that the individual:
- is not engaged in health care services
- is not virally suppressed
- has had a recent STI or
- has recently moved back to NYS from another jurisdiction
In addition, the updated NYS DOH Regulations remove the requirement that data on the partners of HIV cases be destroyed after three years. The NYS DOH or local health department will establish a new policy for record retention and disposition.
Health Care Provider HIV Reporting Requirements
New York State Public Health Law Article 21 requires the reporting of persons with HIV infection and AIDS to the NYS DOH. The law also requires that reports contain the names of sexual or needle-sharing partners known to the medical provider or whom the patient wishes to have notified. Under the federal HIPAA Privacy Rule, public health authorities have the right to collect or receive information "for the purpose of preventing or controlling disease" and in the "conduct of public health surveillance ... " without further authorization. This provision of HIPAA regulations authorizes medical providers to report HIV/AIDS cases to the NYS DOH or NYC Health Department without obtaining patient permission.
The Medical Provider HIV/AIDS and Partner/Contact Report Form (PRF) (DOH-4189) must be completed within 14 days of diagnosis for persons with the following diagnoses or with known sex or needle-sharing partners:
- Initial/New HIV diagnosis – First report of testing documenting HIV diagnosis
- Previously diagnosed HIV (non-AIDS) – Applies to a medical provider who is seeing the patient for the first time
- Initial/New diagnosis of AIDS – Including <200 CD4 cells/μL or an opportunistic infection (AIDS-defining illness)
- Previously diagnosed AIDS – Applies to a medical provider who is seeing the patient for the first time
- Known sex or needle-sharing partners of persons with diagnosed HIV infection
Clinicians seeing for the first time a patient previously diagnosed with HIV or AIDS should report to the NYS DOH using the PRF. The rationale is that this is often the only indication the NYS DOH receives of a patient new to New York, but not newly diagnosed, and perhaps not in need of extensive Health Department Partner Services. Additionally, particularly for the well suppressed patient who moves into NYS, the report by the clinician can be the only indication that the person is in fact HIV positive.
Information regarding electronic reporting via the HIV/AIDS Provider Portal (see below) or paper forms are available from the NYS DOH at 518-474-4284; clinicians located in NYC, call 212-442-3388. To protect patient confidentiality, faxing reports is not permitted.
HIV/AIDS Provider Portal
The HIV/AIDS Provider Portal is an electronic system that enables clinicians to:
- Meet their reporting requirements electronically
- Provide a mechanism for clinicians statewide to notify the NYS DOH that a patient needs linkage to Health Department Partner Services and
- Submit inquiries for patients with diagnosed HIV infection who may need assistance with linkage to or retention in HIV medical care.
A NYS DOH Health Commerce System (HCS) Medical Professionals account is required. After logging into the Health Commerce Systems, select Refresh My Applications List on the left side and then under My Applications select HIV/AIDS Provider Portal. Follow the prompts to set up an account.
Laboratory Reporting Requirements
Laboratory reporting of suspected or confirmed positive findings or markers of HIV infection is mandated under New York State Public Health Law. Guidance has been prepared to assist permitted clinical laboratories and blood banks in meeting their obligations to report HIV-related laboratory test results, as well as other communicable disease markers. The guidance is available on the Wadsworth Laboratory website.
HIV laboratory reporting is an essential source of information for New York's HIV surveillance efforts and maintaining high-quality, complete data is critical to tracking progress toward National HIV/AIDS Strategy retention and care measures and New York's effort to end the epidemic. To keep pace with advances in HIV care, testing technologies and disease monitoring, there have been some important changes to HIV laboratory reporting requirements. Laboratories and blood/tissue banks performing tests for screening, diagnosis or monitoring of HIV infection for NYS residents and/or NYS health care providers (regardless of patient residence) shall report the following laboratory tests or series of tests used in the diagnosis of HIV infection:
- All reactive/repeatedly reactive initial HIV immunoassay results AND all positive, negative, or indeterminate results from all supplemental HIV immunoassays performed under the second or third step in the diagnostic testing algorithm, including HIV-1/2 antibody differentiation assay, HIV-1 Western blot, HIV-2 Western blot, or HIV-1 immunofluorescent assay
- All HIV nucleic acid (RNA or DNA) detection tests (qualitative and quantitative), including tests on individual specimens for confirmation of nucleic acid-based testing (NAT) screening results
- All CD4 lymphocyte counts and percentages, unless known to be ordered for a condition other than HIV
- HIV genotypic resistance testing via the electronic submission of the protease, reverse transcriptase, and integrase nucleotide sequence
- Positive HIV detection tests (culture, P24 antigen)
All HIV-related laboratory reporting, including by NYC providers and for NYC residents, should be made directly to the NYS DOH, submitted electronically via the NYS DOH Electronic Clinical Laboratory Reporting System (ECLRS).
To improve the quality of data, and in keeping with changes that allow for enhanced use of surveillance data to improve linkage and retention in care, laboratories are required to report results using patient identifying, demographic and locating information, as well as the requesting provider and facility ordering the lab test. The 2016 update requires that when labs report HIV-related test results, the following information should be included:
- Patient identifying, demographic, and locating information
- Provider ordering the test and facility name
- Complete provider and facility address and telephone number
- Provider and facility National Provider Identification
For a complete list of this information and instructions on how to report required data elements, please call 518-474-4284 or contact BHAELab@health.ny.gov
In Labor and Delivery Settings, recommendations are:
- Adoption of point-of-care rapid HIV testing in labor and delivery settings
- Availability of expedited HIV test results prior to delivery to allow maximum benefits of intrapartum ARV prophylaxis for the fetus
- Steps to follow when expedited HIV testing yields a preliminary positive result
- Steps to follow when definitive test results indicate HIV infection is present
- Steps to follow when HIV infection has been definitely excluded in the mother
Pregnant women and exposed infants lost-to-care require immediate action for re-engagement. HIV-positive pregnant women and their exposed infants are a priority when identified as lost-to-care and require immediate action for reengagement. Reengagement in care is especially important for HIV-positive pregnant women who are in their third trimester due to possible increasing viral loads from being non-adherent to ART, leading to increased risk of transmitting HIV to their infants. Ensuring exposed infants are engaged in care is critical during the first 4-6 months to ensure appropriate antiretroviral and opportunistic infection prophylaxis, as well as definitive documentation of the infant's HIV infection status.
If routine attempts for re-engagement of the HIV-positive pregnant woman or her exposed or infected infant(s) are not successful, please contact the NYS DOH Perinatal HIV Prevention Program at 518-486-6048 or submit a request via the NYS DOH HIV/AIDS Provider Portal for assistance. New York City providers should call the NYC DOHMH Field Services Unit at 347-396-7601 for assistance with re-engagement of pregnant women.
Records and Reports
- Create and maintain records and reports that are complete, legible, retrievable, and available for review; such records and reports shall include: a comprehensive prenatal care record for each pregnant woman that documents the provision of care and services required by this section and is maintained in a manner consistent with medical record confidentiality requirements
- A comprehensive prenatal care record should be maintained on each client. Entries should be complete, legible, and accurately reflect any of laboratory testing and special procedures
- Records should be maintained in a manner that safeguards confidentiality requirements
- Develop/implement system to track trimester of entry, low birth weight (LBW) infants, number of prenatal visits, postpartum rate of return, number of c/sections, vaginal births after cesarean sections (VBACs), number of women choosing to breastfeed, and number of teen pregnancies
- An annual report should be accurately completed and submitted within the expected time frame
Internal Quality Assurance
- Develop and implement written policies and procedures establishing an internal quality assurance (IQA) program to identify, evaluate, resolve, and monitor actual and potential problems in patient care
- Implement IQA activities focusing on prenatal care within system-wide QA program
- Develop policies/procedures establishing internal quality assurance plan for prenatal care program
- Recommend IQA should be multidisciplinary and review issues such as nutrition, psychosocial, educational methods, care coordination, risk assessment, and HIV services
Have periodic IQA meetings to discuss prenatal issues:
- A documented and filed prenatal chart audit performed periodically on a statistically significant number of current prenatal client records
- An annual written summary evaluation of all components of such audits
- A system for determining patient satisfaction and for resolving patient complaints
- A system for developing and recommending corrective actions to solve identified problems
- A follow-up process to ensure that recommendations and plans of correction are followed
Prenatal chart audits should be performed using 35-40 indicators.
A tool to conduct chart audit should be developed.
Prepare written summary evaluation of audit findings on an annual basis. Maintain audit summary on file. Develop system for determining patient satisfaction with prenatal program and resolving patient complaints. Recommend administering patient satisfaction survey during client's third trimester or at the postpartum visit.
Documentation should include summary reports of chart audit findings; analysis of outcome statistics; analysis of patient satisfaction survey results with recommendations to correct identified problems. All follow-up is done in a timely manner.
Postpartum Services
Coordinate with the neonatal care provider to arrange for the provision of pediatric care services and patient services.
Stress importance of postpartum/pediatric visit to the mother during third-trimester visits. Develop strategies to encourage client to return for postpartum visit (i.e., incentives). Implement missed visit policy for 'no shows’.
A postpartum visit with a qualified health professional shall be scheduled and conducted in accordance with medical needs, ideally between 7-84 days after delivery. For the interim, furnish each woman with a means of contacting the provider in case postpartum questions or concerns arise.
Provide home visits to assess needs (e.g., adjustment to parenting, feeding, etc.) as indicated. Refer to Care Coordination section for additional guidance. Contents of home visit should be documented in the record.
Develop arrangements for client to contact provider between delivery and scheduled postpartum visit.
Postpartum Visit Components
- Identify any medical, psychosocial, nutritional, alcohol treatment, and drug treatment needs of the mother or infant that are not being met;
- Refer the mother, or other infant caregiver to resources available for meeting such needs and assist in meeting such needs where appropriate;
- Assess family planning needs and provide advice and services or referrals where indicated;
- Provide preconception counseling as appropriate and encourage a preconception visit prior to subsequent pregnancies for women who might benefit from such visit;
- Refer infants for preventive and special care;
- Establish a protocol to provide all postpartum components of care (i.e., identify the needs of woman/infant, necessary referrals, family planning, etc.).
Postpartum documentation should include delivery outcome, maternal physical exam, health status of mother/infant including medical, nutritional, and psychosocial needs with referrals.
Use a standardized medical record with postpartum section or separate postpartum visit tool outlining indicated components of care. If you have questions about the High-Risk Maternity Program, please call 888-830-4300.
Supporting documentation is found here:
https://www.health.ny.gov/prevention/nutrition/wic/breastfeeding
https://www.health.ny.gov/health_care/medicaid/standards/perinatal_care
Medicaid Prenatal Guidelines
The New York State Department of Health worked with internal and external stakeholders to develop updated prenatal standards of care for all pregnant women enrolled in Medicaid. Additionally, Highmark has adopted these guidelines for all other lines of business for the maternity program.
These comprehensive changes will improve the quality of prenatal/postpartum care provided to pregnant women. View the Medicaid Prenatal Care Standards for more information.
Highmark has adopted the New York State Department of Health AIDS institute’s guidelines and criteria for medical care of adults, children, and adolescents with HIV infection.
For HIV Guidelines, review hivguidelines.org or nyhealth.gov/diseases/aids.
Confidentiality of HIV-Related Information
Each health care provider is required to develop policies and procedures (P&P) to assure confidentiality of HIV-related information.
P&P must include:
- Initial and annual in-service education of staff and contractors.
- Identification of staff allowed access and limits of access.
- Procedure to limit access to trained staff (including contractors).
- Protocol for secure storage (including electronic storage).
- Procedures for handling requests for HIV-related information.
- Protocols to protect persons with or suspected of having HIV infection from discrimination.
NYS DOH Requirements for HIV Counselling and Testing, and Care of HIV Positive Individuals
Early identification of Human Immunodeficiency Virus (HIV) infection and entry into care can help HIV-infected persons live longer, healthier lives. In addition, identifying infection can help prevent the spread of the disease through education.
The NYS DOH has requirements regarding HIV counseling, testing, and reporting. Established guidelines help increase HIV testing, ensure entry into care, and expand laboratory reporting.
An HIV test is the only way to determine whether someone has HIV. The decision to have an HIV test is voluntary.
All practitioners and providers must comply with the HIV confidentiality provisions of Title 27-F of the New York State Public Health Law.
Routine HIV Testing in Medical Settings
HIV testing should be a routine part of medical care and other services. Recent data indicate that routine HIV testing may be cost effective, even in areas with seroprevalence lower than one percent.
HIV testing MUST be offered to all persons over the age of 13 receiving hospital or primary care services, with limited exceptions noted in the law. The offering must be made to those inpatient persons seeking services in emergency rooms, and persons receiving primary care as an outpatient at a clinic, or from a physician, physicians' assistant, nurse practitioner, or midwife.
Health care providers in NYS are encouraged to routinely discuss HIV with their patients, regardless of their perceived risk. Since many patients may not be comfortable disclosing risk, providers should adopt a low threshold for recommending testing.
There are three exceptions to the requirement to offer HIV testing:
- If the individual...
- is being treated for life-threatening emergency;
- has previously been offered or has previously been tested for HIV (unless otherwise indicated due to more recent risk behavior);
- has been determined by the attending provider to lack mental capacity to consent.
Documentation Requirements
According to the Public Health Law, the following elements pertaining to HIV testing should be documented in the patient medical record:
- The patient was advised that HIV testing is being done;
- If the patient declines the HIV test; and
- For patients with confirmed HIV infection, the name of the provider/facility with whom the follow-up appointment was made.
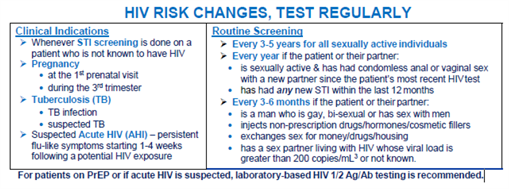
How Often Does the Offer of HIV Testing Need to be Repeated?
In addition to offering HIV testing once in the course of routine care, testing should be offered annually to patients whose behavior indicates elevated risk. To promote early identification, HIV testing may be offered as frequently as every three months to patients with identified risk behaviors. Since many people choose not to disclose their risk behaviors, providers should consider adopting a low threshold for recommending HIV testing.
Requirement for Written or Oral Patient Informed Consent to HIV Testing
Effective November 28, 2016, amendments to the New York State Public Health Law removed the requirement for written or oral informed consent prior to ordering an HIV-related test, including elimination of written consent for HIV testing in New York State correctional facilities, and removing references to consent forms. The objective of the update is to eliminate barriers to HIV testing and make HIV testing comparable to the manner in which other important laboratory tests are conducted. HIV testing remains voluntary and patients have the right to refuse an HIV test, but obtaining written or oral consent for testing is no longer required in any setting. Patients must be advised orally that an HIV test is going to be performed. If the patient objects to the HIV test, this should be noted in the patient's medical record. HIV test requisition forms submitted to laboratories do not require provider certification of informed consent.
Important
Prior to conducting diagnostic HIV testing, information about HIV must be provided orally, in writing, through signage, or in any other patient-friendly, audio-visual format. Placing the NYS DOH HIV testing clinic poster in a visible location or providing patients with the NYS DOH patient brochure on HIV testing are simple ways of conveying this information to patients. The key points of information that must be provided are:
- HIV testing is voluntary, and all HIV test results are confidential (private).
- HIV can be transmitted through unprotected sex, sharing needles, childbirth, or breastfeeding.
- Treatment for HIV is very effective, has few or no side effects, and may involve taking just one pill once a day.
- Partners can keep each other safe by knowing their HIV status and getting HIV treatment or taking HIV pre-exposure prophylaxis (PrEP). Not sharing needles and practicing safer sex will help protect against HIV, hepatitis C, and other STIs.
- It is illegal to discriminate against a person because of his or her HIV status and services are available to help address discrimination.
- Anonymous HIV testing (without giving your name) is available at certain public testing sites.
- HIV testing is a routine part of health care, but you have the right to decline an HIV test; testing will not be performed if you object; if you wish to decline HIV testing, inform the health care provider.
- If the patient declines the offer of an HIV test, this should be noted in the patient's medical record. For patients diagnosed as living with HIV, the health care provider administering testing must arrange, with the consent of the patient, an appointment for HIV medical care; simply providing the name of a care provider is not sufficient; a specific appointment with a provider who offers HIV care should be provided.
These new provisions apply to all HIV testing in New York State and not just for testing as offered to people over the age of 13 in clinical settings.
For additional information, please visit the Department's website by clicking HERE.
Questions may be sent to hivtestlaw@health.ny.gov
Expansion of Minor Consent for HIV Treatment and Preventive Services
The 2017 amendments to NYS DOH's regulations allow minors to consent to their own HIV treatment and HIV preventive services such as pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) without parental/guardian involvement (10 NYCRR Part 23). Part 23 has long established the legal capacity of minors to consent to treatment and preventive services for sexually transmitted diseases (STIs). Provisions in Part 23 require that the Commissioner of Health promulgate a list of sexually transmitted diseases. The 2017 amendments to 10 NYCRR Part 23 added HIV to the list of STIs, thereby bringing minor capacity to consent to HIV treatment and preventive services on par with other STIs. In addition, under Part 23, medical or billing records may not be released or made available to the parent or guardian without the minor patient's permission.
After being diagnosed, young people currently face barriers that can prevent or delay access to care, including denial and fear of their HIV status, misinformation, HIV-related stigma, low self-esteem, lack of insurance, homelessness, substance use, mental health issues, and lack of adequate support systems. Because of these factors, many young people need the ability to consent to their HIV treatment. Updates to regulation help ensure that more young people have optimal health outcomes and prevent transmission of HIV to others. In addition, minors will now have the ability to consent to HIV-related preventive services, including PrEP and PEP just as they can consent for other reproductive or sexual health-related services.
Post-Test Counseling and Requirements to Link Newly Diagnosed Patients to HIV Care
When testing indicates a diagnosis of HIV infection, the person ordering HIV testing or their representative must provide the patient the final interpretation of diagnostic testing, and, with the patient's consent, schedule an appointment for follow-up HIV medical care.
Important
A person with laboratory evidence of acute or early HIV infection (i.e., detectable HIV antigen and virus, but no evidence of HIV antibodies) has a high likelihood of passing the virus to sexual or needle-sharing partners and should be counseled about how to avoid passing the virus to others.
Patient Education That Should be Provided
Patients should be educated on the following:
- That the diagnosis means the person is living with HIV, a lifelong health condition.
- That people can live a healthy life with HIV; HIV treatment is effective, has few or no side effects, and may involve taking just one pill once a day.
- That financial assistance is available, if needed, for HIV medical care and HIV medications.
- That the patient can pass HIV to sexual or needle-sharing partners and strategies for avoiding transmission, including information about mother-to-child transmission.
- The importance of notifying sexual or needle-sharing partners to prevent further transmission and to promote access of exposed persons to HIV testing, health care, and prevention services.
- The range of partner notification options and available partner services programs.
- That names and other information about the patient is not shared during the partner notification process.
- That known contacts, including a known spouse, are reported to the health department.
- The risk of domestic violence and performance of domestic violence screening using the NYS DOH-approved domestic violence screening protocol.
- That HIV-related information is confidential; information may be shared with medical providers to provide needed care but may not be shared with others without patient authorization to release confidential HIV-related information.
- That a minor who has been diagnosed with HIV may consent to their own HIV treatment (if applicable).
- That patient authorization to release confidential HIV-related information may be revoked at any time.
- That discrimination against persons with HIV in the areas of employment, housing, public accommodations, health care, and social services is prohibited by law.
- That all cases of HIV infection are reported to the health department.
- That if a person with HIV appears to be out of care, he or she may be contacted by the medical provider or health department staff to address barriers to entry into care and promote engagement in care.
Important
Undetectable equals Untransmittable (U=U): There are many important reasons to start HIV treatment as soon as possible. In addition to getting treatment to support their own health, a person living with HIV who is on HIV treatment and virally suppressed for 6 months or longer has effectively no risk of passing HIV to a partner through sex.
Persons Who Test Negative for HIV Infection
A person who tests negative for HIV infection must be informed of the result and provided information concerning the risk of acquiring HIV through sexual and needle-sharing activities. Pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) should be discussed as prevention options. This information may be in the form of written materials such as the NYS DOH document titled Information on Non-reactive (Negative) HIV Test Results.
The negative test result and required information do not need to be provided in person. Other mechanisms such as email, mail, and phone may be used as long as there is an established protocol. Alternative methods of delivering results must be discussed with the patient. It is not appropriate to tell patients that if they are not contacted, they may assume their test was negative.
Patients with potential recent exposure to HIV present diagnostic challenges due to the "window period," or the length of time after infection that it takes for antibodies or the virus to be detected by HIV diagnostic tests. More information about the window period for various types of tests can be found here. Clinicians should be familiar with the testing process used by the laboratory conducting testing for their patients because recommendations for retesting patients with recent exposure will vary depending on the test used.
Persons with Inconclusive or Incomplete HIV Diagnostic Testing Results
A person with inconclusive or incomplete HIV diagnostic testing results, i.e., when the HIV Diagnostic Testing Algorithm did not produce an overall valid or conclusive result, shall be informed that the test result was inconclusive or incomplete and have an additional specimen collected as soon as possible. In these cases, the entire algorithm should be repeated. More information is available at: https://www.health.ny.gov/diseases/aids/providers/testing/docs/testing_fact_sheet.pdf
The New York State Health Department may be able to assist if you have difficulty locating a patient in need of additional testing to resolve inconclusive HIV diagnostic testing through Information on Partner Services.
Universal Recommendation for Testing of Pregnant Women
New York's regulatory framework for preventing mother-to-child transmission (MTCT) of HIV has proven highly effective and remains unchanged. The only exception is that the 2017 updates to HIV testing do remove the requirement to obtain consent for HIV testing in writing or orally. All pregnant women must be offered HIV testing as a clinical recommendation as early as possible during pregnancy. Third-trimester testing is recommended for all pregnant women in NYS who tested negative for HIV earlier in their pregnancy. When being offered HIV testing, the woman should be provided the key points of information and informed of her right to decline the test. Pregnant women who are diagnosed as living with HIV should be linked to treatment as soon as possible to protect their health and prevent transmission of HIV to the newborn.
Women who present to the labor/delivery setting with no history of HIV testing during their current pregnancy should be counseled with the recommendation for HIV testing. If the mother declines testing in labor/delivery, the mother should be informed that her newborn will be tested immediately at birth without her consent. All newborns, including those tested at birth, are routinely tested for HIV through the New York State Newborn Screening Program. Documentation of the woman's prenatal HIV testing should be forwarded to the delivering hospital and a copy of the mother's HIV test history results should be placed in the newborn's medical record to ensure administration of medications during labor/delivery and initiation of medication to the infant for the first four-six weeks of life or until the infant is definitively excluded from HIV infection. To access the latest regulations, visit:
Title: Section 69-1.3 - Responsibilities of the CEO of a Hospital
Title: Section 405. 21 - Perinatal services
Acute HIV Infection During Pregnancy
The acute HIV infection in pregnancy guidelines recommend the following:
- Confirmation of preliminary positive expedited HIV test results;
- Vigilance for acute HIV infection in pregnant women who present with a compatible clinical syndrome, even if a previous HIV antibody test during current pregnancy was negative;
- Evaluation for acute HIV infection in pregnant or breastfeeding women who present with a febrile "flu" or "mono" like illness, or rash that is not otherwise explained;
- Immediate screening for suspected acute HIV infection by obtaining an HIV serologic screening test in conjunction with a plasma HIV RNA assay (a fourth-generation HIV antigen/antibody combination test is the preferred serologic screening test, if available);
- Repeat HIV RNA testing from a new specimen to confirm the presence of HIV RNA if HIV RNA or antigen was detected in the absence of HIV antibody; and
- Baseline genotypic testing and initiation of ART while waiting for the results of resistance testing.
Rapid Test Technology
Rapid HIV antibody tests that can provide a preliminary* result during a single appointment are recommended. Individuals may be more likely to be tested for HIV if they know that the appointment, inclusive of counseling, consent, and testing, will be relatively brief.
*Further testing is always required to confirm a reactive (preliminary positive) screening test result.
Consent for rapid HIV testing can be oral and noted in the medical record, including:
- Offering of testing during labor and delivery for those who do not have documented third trimester HIV test results; and
- Availability of expedited testing of pregnant women who present for delivery without documentation of a negative HIV test.
In Labor and Delivery Settings, recommendations are:
- Adoption of point of care rapid HIV testing in labor and delivery settings;
- Availability of expedited HIV test results prior to delivery to allow maximum benefits of intrapartum ARV prophylaxis for the fetus;
- Steps to follow when expedited HIV testing yields a preliminary positive result;
- Steps to follow when definitive test results indicate HIV infection is present; and
- Steps to follow when HIV infection has been definitely excluded in the mother.
Additional information about rapid testing is available at the Department of Health's: HIV Testing
Additional Material:
AIDS Institute NYS DOH Counseling and Testing Resources
The following numbers are available to call for HIV information, referrals, or information on how to obtain a free HIV test without having to give the client's name and without waiting for an available appointment:
- Counseling/Testing: 800-541-AIDS
Special initiatives are available to providers who want to arrange for a program presentation or possible anonymous HIV counseling and testing at their sites. Providers should contact the regional coordinator of the Anonymous HIV Counseling and Testing Program at the appropriate toll-free number listed above.
NYSDOH AIDS Institute Resource Directory
The NYS DOH AIDS Institute has a resource directory intended for use by individuals seeking services and as a referral tool for providers. This directory is arranged by region, with each organization listed under the region it services, and then by the service(s) it provides. This directory can be found at the Department of Health.
Partner Services and the Role of Partner Services Program
Medical providers or their designee must explain to all newly diagnosed patients the importance of notifying any sexual or needle-sharing partners that they may have been exposed to HIV. Partner services is a cornerstone of HIV prevention efforts that provides an opportunity for sexual or needle-sharing contacts of a person living with HIV to be offered testing in a timely manner, and if diagnosed with HIV infection, be linked into care. Every physician or other person authorized to order diagnostic testing is required to report HIV and AIDS diagnoses to the health department. This report must include identifying information about any contacts known to the clinical provider or provided to the clinical provider by the patient. The HIV/AIDS Provider Portal may be used to report cases (including partners) and to request assistance from the health department with partner notification. As part of post-test counseling, the following must be provided to the patient:
- An explanation of the importance of notifying sexual or needle-sharing partners to prevent further transmission, and to promote early access of exposed persons to HIV testing, health care, and prevention services.
- A description of notification options and assistance available to the protected individual.
- A discussion about the risk of domestic violence and screening for domestic violence prior to partner notification in accordance with NYS DOH domestic violence screening protocol.
- The fact that known contacts, including a known spouse, will be reported to the health department; that protected persons will also be requested to cooperate in contact notification efforts of known contacts and that protected persons may name additional contacts they wish to have notified with the assistance of the provider or authorized public health officials.
- An explanation that the name and other information about the person living with HIV will be protected during the contact notification process.
The NYS DOH Partner Services Program and the New York City (NYC) Health Department Contact Notification Assistance Program (C-NAP) provide a wide range of services, including performing notifications; assisting patients with decision making; and consulting with health care providers. In some situations, Partner Services specialists can meet with the patient at the same time that the laboratory results are given to assist with post-test counseling and development of a partner notification plan. Additional NYS DOH/NYC Department of Health and Mental Hygiene (NYCHMH) services may be available, such as assistance in locating persons who test positive but who do not return for their results. For more information about partner services and how to contact partner services programs throughout NYS, visit: Information on Partner Services.
Important
In recognition of the need for ongoing partner services beyond the time of initial diagnosis of HIV, the 2016 updates to the NYS DOH Regulations formally prioritized partner services for people who were previously diagnosed with HIV and who are at elevated risk of transmitting the virus to others. Several factors are considered as evidence of elevated risk of transmitting the virus to others. Those factors include that the individual:
- Is not engaged in health care services;
- Is not virally suppressed;
- Has had a recent STI; or
- Has recently moved back to NYS from another jurisdiction.
In addition, the updated NYS DOH Regulations remove the requirement that data on the partners of HIV cases be destroyed after three years. The NYS DOH or local health department will establish a new policy for record retention and disposition.
Health Care Provider HIV Reporting Requirements
New York State Public Health Law Article 21 requires the reporting of persons with HIV infection and AIDS to the NYS DOH. The law also requires that reports contain the names of sexual or needle-sharing partners known to the medical provider or whom the patient wishes to have notified. Physicians and others authorized to order HIV testing are required to report any determination or diagnosis of HIV infection, including primary HIV infection, acute retroviral syndrome, and early HIV infection, within one day (24 hours) of such determination or diagnosis. Insurance institutions, insurance support organizations, and health care providers associated with or under contract to a health maintenance organization or other medical services plan are subject to these regulations, except as noted in section 63.6(a)(9), (10), and (12).
The Medical Provider HIV/AIDS and Partner/Contact Report Form (PRF) (DOH-4189) must be completed no later than seven days after the provider’s receipt of a positive laboratory result or after diagnosis, whichever is sooner, for persons with the following diagnoses:
- Initial/New HIV diagnosis – First report of HIV antigen/antibody positive test results;
- Previously diagnosed HIV infection (non-AIDS) – Applies to a medical provider who is seeing the patient for the first time;
- Initial/New diagnosis of AIDS – Including <200 CD4 cells/μL or an opportunistic infection (AIDS-defining illness);
- Previously diagnosed AIDS – Applies to a medical provider who is seeing the patient for the first time;
- Known sex or needle-sharing partners of persons with diagnosed HIV infection.
Medical providers must complete the NYS Medical Provider HIV/AIDS and Partner/Contact Report Form (DOH-4189) for all reportable cases. For information regarding electronic reporting or paper forms, please call the NYS DOH at 518-474-4284. Completed paper Provider Report Forms for practitioners outside of NYC can be mailed to:
Division of Epidemiology, Evaluation, and Partner Services
P.O. Box 2073, ESP Station
Albany, NY 12220-0073
For clinicians located in NYC, please call 212-442-3388. To protect patient confidentiality, faxing of reports is not permitted.
HIV/AIDS Provider Portal
The HIV/AIDS Provider Portal is an electronic system that enables clinicians to:
- Meet their reporting requirements electronically
- Provide a mechanism for clinicians statewide to notify the NYS DOH that a patient needs linkage to Health Department Partner Services and
- Submit inquiries for patients with diagnosed HIV infection who are thought to be in need of assistance with linkage to or retention in HIV medical care.
After logging into the Health Commerce System, select Refresh My Applications List on the left side and then under My Applications select HIV/AIDS Provider Portal. Follow the prompts to set up an account.
Laboratory Reporting Requirements
Laboratory reporting of suspected or confirmed positive findings or markers of HIV infection is mandated under New York State Public Health Law. Guidance has been prepared to assist permitted clinical laboratories and blood banks in meeting their obligations to report HIV-related laboratory test results, as well as other communicable disease markers. The guidance is available here.
HIV laboratory reporting is an essential source of information for New York's HIV surveillance efforts and maintaining high quality, complete data is critical to tracking progress toward National HIV/AIDS Strategy retention and care measures and New York's effort to end the epidemic. To keep pace with advances in HIV care, testing technologies and disease monitoring, there have been some important changes to HIV laboratory reporting requirements.
Laboratories and blood/tissue banks performing tests for screening, diagnosis, or monitoring of HIV infection for NYS residents and/or NYS health care providers (regardless of patient residence) shall report the following laboratory tests or series of tests used in the diagnosis of HIV infection:
- All reactive/repeatedly reactive initial HIV immunoassay results AND all positive, negative, or indeterminate results from all supplemental HIV immunoassays performed under the second or third step in the diagnostic testing algorithm, including HIV-1/2 antibody differentiation assay, HIV-1 Western blot, HIV-2 Western blot, or HIV-1 immunofluorescent assay.
- All HIV nucleic acid (RNA or DNA) detection tests (qualitative and quantitative), including tests on individual specimens for confirmation of nucleic acid-based testing (NAT) screening results.
- All CD4 lymphocyte counts and percentages, unless known to be ordered for a condition other than HIV.
- HIV genotypic resistance testing via the electronic submission of the protease, reverse transcriptase, and integrase nucleotide sequence.
- Positive HIV detection tests (culture, P24 antigen).
All HIV-related laboratory reporting, including by NYC providers and for NYC residents, should be made directly to the NYS DOH, submitted electronically via the NYS DOH Electronic Clinical Laboratory Reporting System (ECLRS).
To improve the quality of data, and in keeping with changes that allow for enhanced use of surveillance data to improve linkage and retention in care, laboratories are required to report results using patient identifying, demographic and locating information, as well as the requesting provider and facility ordering the lab test. The 2016 update requires that when labs report HIV-related test results, the following information should be included:
- Patient identifying, demographic, and locating information
- Provider ordering the test and facility name;
- Complete provider and facility address and telephone number; and
- Provider and facility National Provider Identification.
For a complete list of this information and instructions on how to report required data elements, please call 518-474-4284 or contact BHAELab@health.ny.gov
Care of HIV Positive Individuals
The NYS DOH AIDS Institute’s clinical guidelines pertaining to HIV prevention and the medical management of adults, children, and adolescents with HIV infection can be found on the Department of Health AIDS Institute website.
All clinical care settings should be prepared, either on-site or with a confirmed referral, to support patients in initiating antiretroviral therapy (ART) as rapidly as possible after diagnosis.
A new HIV diagnosis is an immediate call to action for every provider who engages with that individual, to assure the rapid initiation of antiretroviral treatment (RIA). New York State Department of Health (NYS DOH) HIV Clinical Guidelines state that treatment is recommended for all patients with a confirmed HIV diagnosis regardless of their CD4 cell count or viral load. All providers serving persons with HIV should establish systems which strive for the same-day initiation of HIV treatment, even while initial lab work is pending. While same-day initiation of treatment may not always be possible, it is ideal that patients be started on treatment within three days. In the outpatient setting, in no instance should treatment initiation take longer than 30 days.
On April 1, 2014, Public Health Law Section 2135 was amended to promote linkage and retention in care for HIV-positive persons. The law allows the NYS DOH and New York City Department of Health and Mental Hygiene (NYC DOHMH) to share information with health care providers for the purposes of patient linkage and retention in care. The NYS DOH AIDS Institute recommends that health care providers take a multi-pronged approach to support their patients' retention in care, including but not limited to the following:
- Have a proactive patient plan: do not wait for a lapse in care to discuss what to do if the patient becomes lost-to-care.
- Create a patient-centered atmosphere, where all members of the medical care teams (e.g., reception staff, phlebotomists, medical providers) promote patient engagement, linkage, and retention in care.
- When acceptable to patients, expand authorization dates on Authorization for Release of Health Information and Confidential HIV-Related Information forms (DOH-2557) to at least two years. Extending consent timeframes allows collaboration across sectors. Have DOD-2557 consent forms on file for every patient. This will permit you to contact community-based organizations (CBOs) and others in the event of a lapse in care. Examples of CBOs that can help return patients to care include but are not limited to: HIV/AIDS CBOs: Health Homes and their downstream providers; food and nutrition programs; shelters; substance use treatment facilities; housing providers; mental health providers; prenatal care providers, etc.
- Encourage patients to add your practice's name to any releases they sign with other organizations.
- Work with patients to update releases prior to when the releases expire (if applicable).
- Become a member of your area's Health Home network(s) if you have not already done so, visit Find A Health Home.
Leverage Existing Resources for Patient Re-Engagement
- Use information from the Regional Health Information Organization (RHIO), if available, to determine if the patient is in care with another provider or if updated personal contact information is available.
- Conduct a health insurance benefits check, if available, on the patient to determine if he/she changed insurance or is in care with another provider.
- If the patient is in a Managed Care plan, the plan will have updated contact information, recent use of care, and medications on file. If this is a Medicaid Managed Care Plan, the plan can identify which Health Home the patient may be enrolled in as this information may be useful to your follow-up efforts.
- If your patient is enrolled in a Health Home and has signed a release, contact the Health Home to determine whether the patient is actively enrolled. If yes, request assistance to contact or re-engage the patient in care.
- If your patient has Medicaid but has not been enrolled in a Health Home, contact the Health Home to make an "upstream referral." The patient will be referred to a provider who may conduct outreach to the patient's home.
- Try multiple modes of contact (phone, text, letter, email, and social media) at varying times of the day/week to reach the patient (special consideration for social media sites - contact patient from an agency social media account and not a staff person's personal account).
- If your patient uses other services within the facility (e.g., WIC, dental, child's provider), place an alert on the Electronic Medical Record (EMR) to reconnect to the HIV Primary Care Provider and, if pregnant, to her prenatal care provider.
- As authorized in patient releases and/or medical charts, work with emergency contacts and other agencies/providers to determine whether they have had recent patient contact.
- Conduct a home visit if resources allow. If you have a peer program, utilize peers to provide outreach to the patient's home.
Use External Systems to Expand Your Search When You Cannot Find a Patient
Review public records such as:
- New York Free Public Records Directory (property tax rolls, municipal tax rolls, etc.)
- Parole Lookup
- NYS County Jail Inmate Lookup
- NYC Department of Corrections Inmate Lookup
- NYS Department of Corrections and Community Supervision Inmate Lookup
- Consider using people search engines, local newspapers, and police blotters.
- National Program of Cancer Registries
- A user ID and password are required to access the site and may be obtained by calling 301-572-0502.
Pregnant Women and Exposed Infants Lost-to-Care Require Immediate Action for Re-Engagement
HIV-positive pregnant women and their exposed infants are a priority when identified as lost-to-care and require immediate action for re-engagement. Re-engagement in care is especially important for HIV-positive pregnant women who are in their third trimester due to possible increasing viral loads from being non-adherent to ART, leading to increased risk of transmitting HIV to their infants. Ensuring exposed infants are engaged in care is critical during the first 4-6 months to ensure appropriate antiretroviral and opportunistic infection prophylaxis, as well as definitive documentation of the infant's HIV infection status.
If routine attempts for re-engagement of the HIV-positive pregnant woman or her exposed or infected infant(s) are not successful, please contact the NYS DOH Perinatal HIV Prevention Program at 518-486-6806 or submit a request via the NYS DOH HIV/AIDS Provider Portal (see below) for assistance. NYC providers should call the NYC DOHMH Field Services Unit at 347-396-7601 for assistance with re-engagement of pregnant women.
Providers in NYS Based Outside of NYC
After exploring the investigation tools and strategies listed above and if patient follow-up is warranted, the Bureau of HIV/AIDS Epidemiology (BHAE) may be able to provide information regarding a patient's care status through the NYS DOH HIV/AIDS Provider Portal. The HIV/AIDS Provider Portal is an electronic system which enables clinicians to:
- Meet their reporting requirements electronically;
- Provide a mechanism for clinicians statewide to notify the NYS DOH that a patient needs linkage to Health Department Partner Services; and
- Submit inquiries for patients with diagnosed HIV infection who are thought to be in need of assistance with linkage to or retention in HIV medical care.
A NYS DOH Health Commerce System (HCS) Medical Professionals account is required. To apply for an HCS Medial Professions account, visit NYS Department of Health.
Pre-Exposure Prophylaxis (PReP)
In 2017, the Centers for Disease Control and Prevention (CDC) released its guidelines for the use of daily pre-exposure prophylaxis (PrEP) for the prevention of HIV infection. The following CDC PrEP documents are available:
The New York State Health Department urges providers to adhere to CDC and New York State guidelines with their patients on PrEP by:
- Testing for HIV every three months using a laboratory-based, ideally 4th generation HIV test;
- Assessing for signs of acute HIV infection at every visit;
- Having a low threshold for testing for acute HIV and STIs; and
- Encouraging patients on PrEP (or on HIV treatment) to use condoms as often as possible.
Visit Pre Exposure Prophylaxis (PrEP) and Post-Exposure Prophylaxis (PEP) for more information.
Reporting Suspected Seroconversion
Providers who manage patients on PrEP are strongly encouraged to immediately report any cases of suspected PrEP/PEP breakthrough HIV infection as follows:
- NYC: Report cases to the New York City Department of Health and Mental Hygiene by calling 212-442-3388
- Remainder of state: Report cases to New York State Department of Health by calling 518-474-4284 or using DOH-4189 and contacting the local Partner Services Program to discuss the case.
State law requires that providers report all cases of HIV infection as soon as possible but no later than seven days after diagnosis. Rapid case reporting is critical, because it allows health departments to investigate the case and engage field staff to:
- Conduct outreach to the patient's social network;
- Make HIV testing available to exposed partners; and
- Reduce secondary transmission by expediting linkage to care and PrEP/PEP referrals.
Tuberculosis Facts and Internet Resources
Tuberculosis (TB) is a bacterial disease usually affecting the lungs. TB is spread through the air and can affect anyone of any age. Treatment can be complicated and often includes taking medication for three to nine months.
According to the Blue Cross and Blue Shield Association, Blue Cross and Blue Shield companies work with more than 90 percent of all doctors and hospitals nationwide and, therefore, have a unique perspective on doctors and hospitals that are effective in improving patient care and health. This perspective is the foundation of Blue Distinction®, the Blue Cross and Blue Shield national doctor and hospital recognition program. These recognized doctors and hospitals are changing health care to be more patient-focused, coordinated, and in many cases, affordable.
Blue Distinction designations are based on strict performance criteria formulated through insights and recommendations from the medical community to be consistent with medical advances and current clinical practices, guidelines, and measurement. The aim of the Blue Distinction designation is to help Blue Plan members find the highest quality care available in their area. The Blue Distinction portfolio includes three programs:
- Specialty Care recognizes providers that demonstrate proven expertise in delivering effective and cost-efficient care for select specialty areas. The program targets procedures and episodes of care in areas of high or increasing demand, yet with variations in quality and cost.
- Total Care recognizes providers participating in locally tailored programs (Patient-Centered Medical Homes, Accountable Care Organizations, or similar programs) designed to lower cost trend through better coordinated care and performance-based payment.
- Flexible Network is the nation’s largest custom-tiered network solution, enabling accounts to achieve the optimal balance of savings and employee access via customizable benefit levels.
Blue Distinction Specialty Care
The Specialty Care Program relies on objective, nationally consistent quality and affordability criteria, enabling Blue Cross and Blue Shield Plans to recognize providers that demonstrate expertise in delivering quality specialty care effectively and cost-efficiently.
The foundation of Specialty Care is the quality-focused Blue Distinction Center designation. An additional and more select value-based designation, Blue Distinction Center+, further distinguishes providers delivering quality, cost-efficient specialty care. Quality is key: only those providers that first meet Blue Distinction Centers’ objective, nationally consistent quality criteria are considered for designation as a Blue Distinction Center+.
The Blue Distinction Specialty Care Program includes two levels of designation:
- Blue Distinction Centers are health care facilities recognized for their expertise in delivering quality specialty care safely and effectively.
- Blue Distinction Centers+ are health care facilities recognized for their expertise and efficiency in delivering specialty care. To earn this designation, hospitals must meet the same quality criteria as Blue Distinction Centers, and then go an extra step to demonstrate they do so efficiently (i.e., cost of care measures).
Blue Distinction Specialty Care has 11 areas of specialty care:
- Bariatric surgery
- Maternity care
- Cardiac care
- Spine surgery
- Cancer care
- Transplants
- Knee and hip replacement
- Fertility care
- Gene therapy (ocular disorders)
- Cellular immunotherapy (CAR-T)
- Substance use treatment and recovery
Blue Distinction Total Care
Blue Distinction Total Care is a national program that recognizes doctors who spend more time on prevention, holistic (“total”) care, and personalized care planning for their patients. Total Care encourages a focus on health care instead of sick care. The program is designed to encourage strong relationships between doctors and their patients that can lead to better health.
Designation as a Blue Distinction Total Care provider means this provider has met the established national criteria and has been designated by the local plan. Blue Cross and Blue Shield companies nationwide use the same criteria to select programs for Blue Distinction Total Care.
Blue Distinction Specialty Care Benefit Designs
Highmark offers health plans that may include a benefit for Blue Distinction Specialty Care. Members may be able to reduce their out-of-pocket costs by receiving quality care in any one of five specialty areas at Blue Distinction Center and/or Blue Distinction Center+ providers.
These Blue Distinction service-based plans recommend Blue Distinction Centers/Centers+ for the following specialty care: bariatric surgery, cardiac care, knee and hip replacement, spine surgery, and transplants. These benefit plans may also include a travel and lodging benefit for members who may have to travel beyond a specified mileage limit to access a Blue Distinction Center.
To assure Highmark members receive the highest quality specialty care, professional providers are encouraged to recommend facilities that have received Blue Distinction designation. As you verify a member’s eligibility and benefits prior to rendering services and making recommendations for specialty care, please be sure to verify if the member’s coverage includes a benefit for Blue Distinction Specialty Care.
Locating Blue Distinction Recognized Providers
Information on Blue Distinction designations for participating physicians and hospitals is available in the Highmark Provider Directory. The Provider Directory is accessible on the home page of Highmark.com. Click on Accreditations on the provider’s profile page to determine if a physician has received the Blue Distinction Total Care designation or the facility has received Blue Distinction Center and/or Blue Distinction Center+ designation(s) for any of the seven types of specialty care.
BCBSA Blue Distinction Center Finder
To locate Blue Distinction Centers and Blue Distinction Centers+ near you or in other locations, you can also use the Blue Cross and Blue Shield Association’s Blue Distinction Center Finder.
The following entities, which serve the noted regions, are independent licensees of the Blue Cross Blue Shield Association: Western and Northeastern PA: Highmark Inc. d/b/a Highmark Blue Cross Blue Shield, Highmark Choice Company, Highmark Health Insurance Company, Highmark Coverage Advantage Inc., Highmark Benefits Group Inc., First Priority Health, First Priority Life, Highmark Care Benefits Inc., or Highmark Senior Health Company. Central and Southeastern PA: Highmark Inc. d/b/a Highmark Blue Shield, Highmark Benefits Group Inc., Highmark Health Insurance Company, Highmark Choice Company or Highmark Senior Health Company. Delaware: Highmark BCBSD Inc. d/b/a Highmark Blue Cross Blue Shield. West Virginia: Highmark West Virginia Inc. d/b/a Highmark Blue Cross Blue Shield, Highmark Health Insurance Company or Highmark Senior Solutions Company. Western NY: Highmark Western and Northeastern New York Inc. d/b/a Highmark Blue Cross Blue Shield. Northeastern NY: Highmark Western and Northeastern New York Inc. d/b/a Highmark Blue Shield.
All references to “Highmark” in this document are references to the Highmark company that is providing the member’s health benefits or health benefit administration and/or to one or more of its affiliated Blue companies.
All revisions to this Highmark Provider Manual (the “manual” or “Highmark Provider Manual”) are controlled electronically. All paper copies and screen prints are considered uncontrolled and should not be relied upon for any purpose.